Cell Metabolism: The mechanism of protecting liver and metabolism by exercise has finally been clarified.
The original Singularity Cake Singularity Network is included in the collection # 50 Sports.
* For medical professionals only.


In addition to improving body muscle shape, exercise is more an effective health intervention, which is related to the reduction of the risk of chronic metabolic diseases such as obesity, diabetes and cardiovascular diseases.
However, it is easy to say that "exercise is good for physical and mental health", but it is difficult to explain the mechanism. There are still many unsolved mechanisms of exercise on systemic metabolic health. For example, a recent study shows that, but researchers have not directly proved the specific benefits of exercise to the liver [1].
This time, the team of He Congcong from Northwestern University found that the initiation of liver autophagy is a key link for exercise to bring systemic metabolic benefits.
Their research shows that exercise causes muscle to release a signal molecule called fibronectin 1(FN1), which reaches the liver through blood circulation and acts on α5β1 integrin on the surface of liver cells, thus triggering autophagy in the liver, thus improving metabolic health problems such as insulin sensitivity [2]. The article was recently published in the journal Cell Metabolism.

Screenshot of the first page of the paper
Autophagy abnormality has been considered to be related to many metabolic disorders in recent years. Previous studies have shown that exercise can induce autophagy in skeletal muscle of mice or humans through energy stress, oxidative stress and increase of intracellular Ca2+ level. In addition to these muscle tissues, many studies have shown that exercise can also activate autophagy in non-contractile muscle tissues such as liver, pancreas and adipose tissue, but the mechanism is still unclear [2].
Therefore, He Congcong and others deeply explored whether exercise can unlock the autophagy pathway of the liver and its role in systemic metabolic health.
First of all, they constructed a group of mouse models (ATG7Δliver) in which autophagy gene ATG7 was specifically down-expressed, so as to inhibit autophagy in liver cells.
Under the regular diet, the metabolic indexes of these mice are similar to those of wild-type mice. However, in the case of high-fat diet and running for 50 minutes every day, after 7 weeks, although the weight of the two groups of mice was similar, unlike wild-type mice, exercise did not save the glucose tolerance and insulin sensitivity of ATG7Δliver mice under high-fat diet.
These indicate that the improvement of metabolic health by exercise depends on autophagy of liver cells.
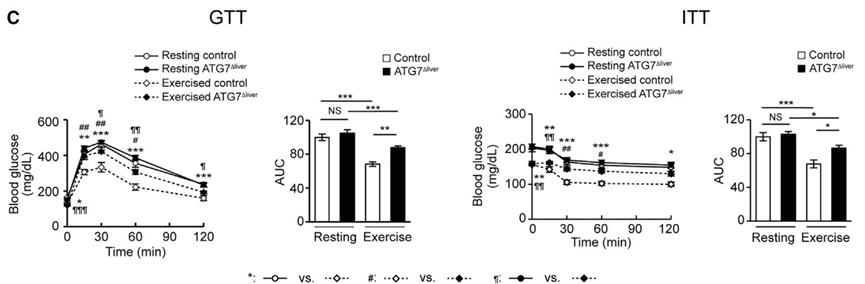
Exercise can’t save at G7 δ liver mice.
The results of in vitro experiments show that plasma and serum of wild-type mice can activate hepatocyte autophagy when they are in exercise, but plasma or serum at rest can not. In other words, there is a switch in the blood to turn on the autophagy pathway of the liver.
After protein’s proteomic analysis of the blood of mice, the researchers found that the level of fibronectin (FN1), an extracellular matrix protein that can be expressed by many types of cells, increased significantly in the blood of wild-type mice after exercise.
In addition, the experimental results in vitro showed that adding activator of AMPK, a cell energy sensor, to the culture medium could induce muscle cells to secrete FN1, but could not induce liver cells to secrete FN1. This suggests that the level of FN1 in blood is regulated by AMPK, and exercise may activate AMPK in muscle.
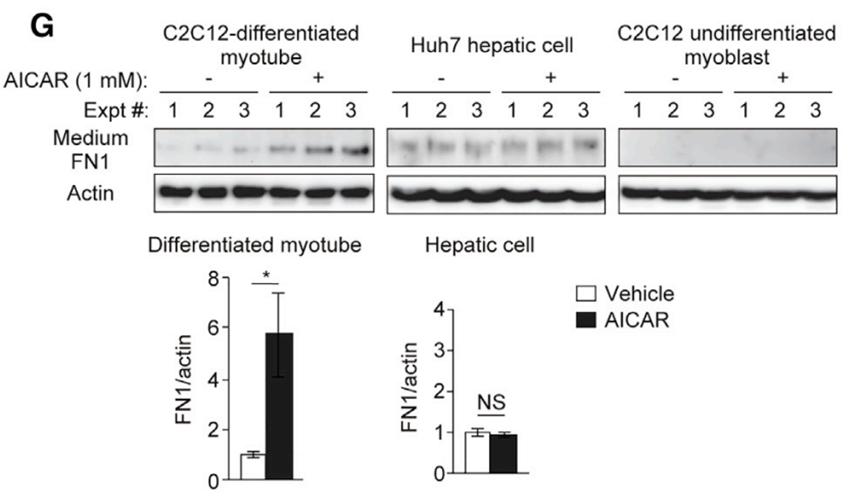
In the exercise state (when AMPK is activated), the extra FN1 comes from muscle cells, not liver cells.
In order to further study the role of muscle-derived FN1 in exercise, researchers specifically depleted FN1 (FN1(FN1Δmuscle) in mice muscles.
The results showed that the level of FN1 in the blood circulation of FN1Δmuscle mice did not increase after wheel running. Compared with wild-type mice, these mice did not get the improvement of systemic insulin sensitivity caused by exercise.
Specifically, the exhaustion of muscle-specific FN1 in mice did not affect its exercise-induced muscle autophagy, muscle morphology and exercise ability, and at the same time, the improvement of exercise-induced insulin signal transduction (activation of Akt regulatory factors) could be observed in its muscles. However, in this group of mice, the exercise-induced liver autophagy pathway was not activated, and no improvement in exercise-induced insulin signal transduction was observed in the liver.
These indicate that FN1 released by muscle is very important for the initiation of liver autophagy during exercise.
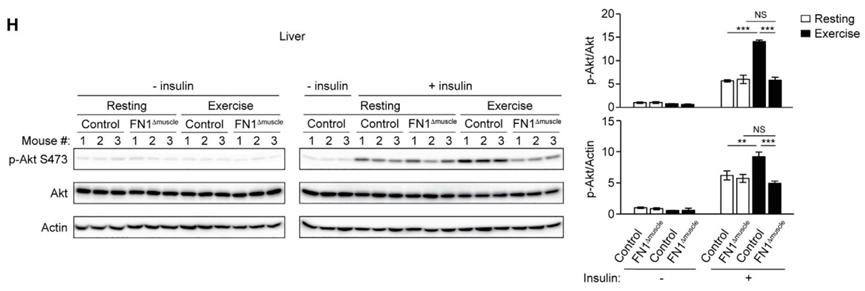
The depletion of FN1 in muscle affects liver autophagy.
From the mechanism point of view, during exercise, muscles secrete FN1, which leads to the increase of FN1 level in blood circulation. FN1 binds and interacts with α5β1 integrin in liver, activating IKK-JNK1-BECN1 signal pathway in liver cells, thus starting liver autophagy, promoting liver autophagy and reducing liver fat accumulation.
If the expression of α5β1 integrin in the liver of mice is inhibited, the weight, glucose tolerance and insulin sensitivity of mice can not be improved after high-fat diet and exercise, which proves the importance of α5β1 integrin -IKK-JNK1 pathway for the benefit of exercise-induced metabolism.
Overall, this study completed by He Congcong’s team shows that liver autophagy plays a key role in exercise-induced metabolic benefits, and reveals its mechanism. In addition, this study also shows that the metabolism of non-contractile muscle tissues such as liver can also be regulated by muscles.
References:
[1]Stine, J. G., DiJoseph, K., Pattison, Z., Harrington, A., Chinchilli, V. M., Schmitz, K. H., & Loomba, R. (2022) . Exercise Training Is Associated With Treatment Response in Liver Fat Content by Magnetic Resonance Imaging Independent of Clinically Significant Body Weight Loss in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. The American journal of gastroenterology, 10.14309/ajg.0000000000002098. Advance online publication. https://doi.org/10.14309/ajg.0000000000002098
[2]https://www.sciencedirect.com/science/article/abs/pii/S1550413123000116


The author of this article is Zhang Aidi
Read the original text